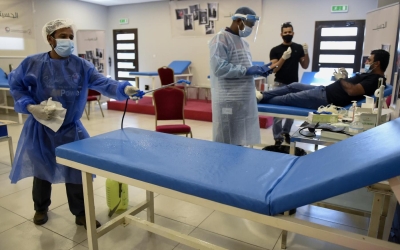
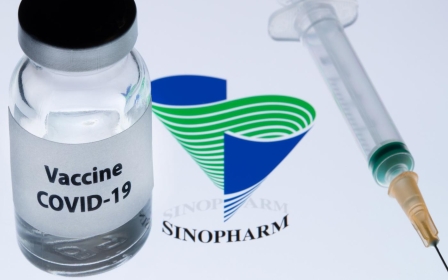
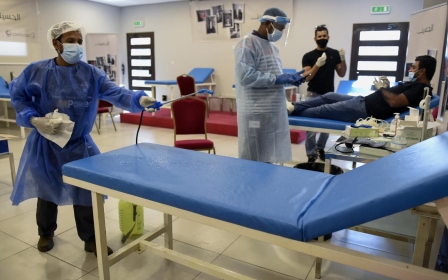
Covid-19: Bahrain approves vaccine from Chinese drug giant Sinopharm
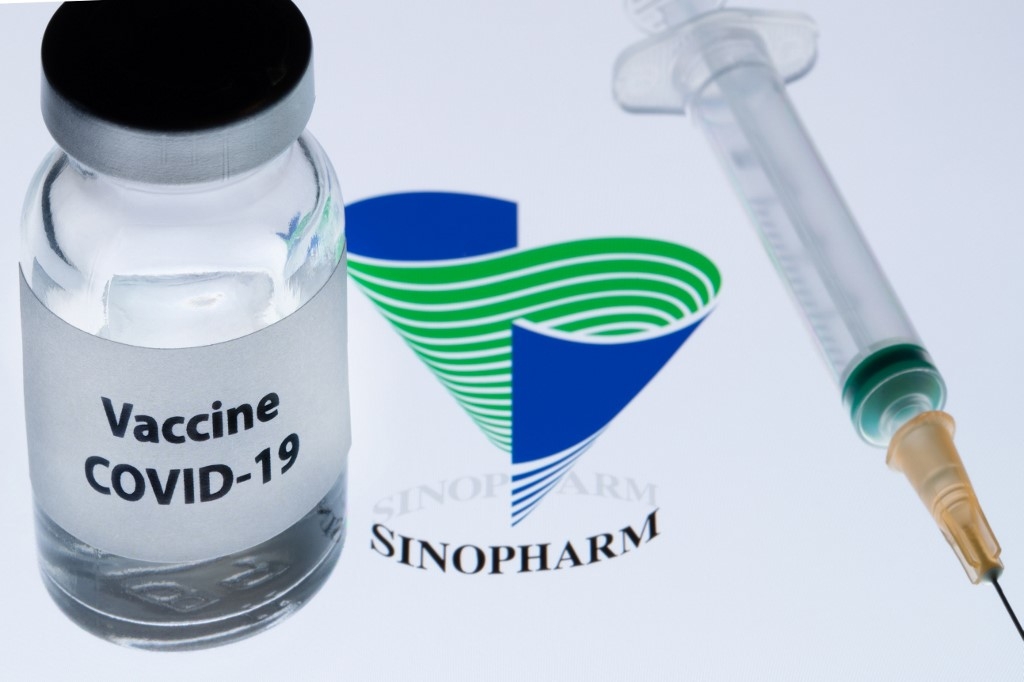
Bahrain said on Sunday that it had approved a Covid-19 vaccine developed by China National Pharmaceutical Group (Sinopharm) and had launched a registration scheme online for the vaccine for citizens and residents, according to Reuters.
A statement by the National Health Regulatory Authority did not specify which vaccine of two being developed by Sinopharm had been given the go-ahead, but cited data from phase-three clinical trials that showed an 86% efficacy rate and said that Bahrain had participated.
Bahrain's Ministry of Health said in a statement and on its Instagram account that citizens and residents above 18 years of age could register online to receive the vaccine for free.
The data cited was the same as announced earlier this month by the United Arab Emirates from an interim analysis of late-stage clinical trials for an inactivated vaccine developed by the Beijing Institute of Biological Product, a unit of Sinopharm's China National Biotec Group (CNBG).
In July, the UAE had started phase-three clinical trials for the Sinopharm vaccine and it was approved for emergency use for healthcare workers in September. The trial had been expanded to Bahrain, Jordan and Egypt.
On 9 December, the UAE's health minister had announced the "official registration" of the Sinopharm vaccine, state news agency WAM said, without elaborating on how it would be used.
"The announcement is a significant vote of confidence by the UAE’s health authorities in the safety and efficacy of this vaccine," WAM said.
"The analysis also shows the vaccine to have a 99 percent seroconversion rate of neutralising antibodies and 100 percent effectiveness in preventing moderate and severe cases of the disease.
"Furthermore, the analysis shows no serious safety concerns."
Four vaccines in development
China has four vaccine products in the final stages of development, three of which - including Sinopharm - use an inactivated form of the novel coronavirus to boost immunity.
This means they only need to be refrigerated and can be easily distributed, compared to jabs developed by rivals Pfizer and BioNTech or Moderna, which have reported efficacy of 95 percent and 94 percent respectively, but which need to be transported at minus 70 degrees Celsius and minus 20 degrees Celsius.
Neither CNBG nor Sinopharm was available for comment.
The Bahrain statement said the kingdom had participated in phase-three trials of the approved vaccine and had previously authorised it for emergency use to frontline professionals.
Bahrain earlier this month granted emergency use authorisation for the Pfizer/BioNTech Covid-19 vaccine.
In fellow Gulf state Kuwait, the health ministry on Sunday granted emergency use authorisation for the Pfizer/BioNTech vaccine, state news agency Kuna said.
Middle East Eye delivers independent and unrivalled coverage and analysis of the Middle East, North Africa and beyond. To learn more about republishing this content and the associated fees, please fill out this form. More about MEE can be found here.